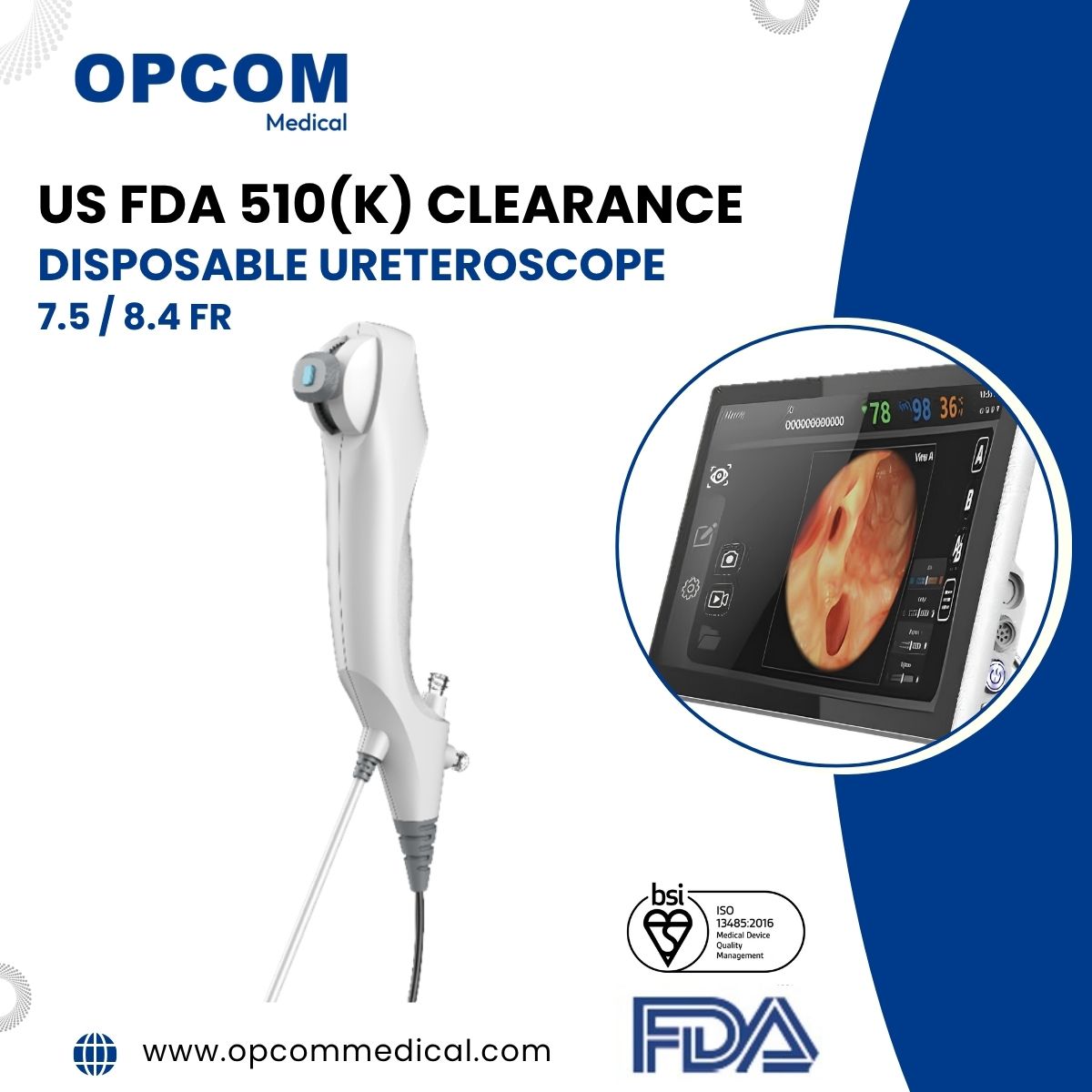
OPCOM Medical Receives The US FDA Approval for Flexible Ureteroscope
We’re pleased to announce today that OPCOM Medical Inc. has received its first approval order from the U.S. Food and Drug Administration (FDA) for its flexible ureteroscope system. This approval marks OPCOM Medical as the first company in Taiwan authorized to manufacture single-use flexible ureteroscopes.
Key Feature
The Disposable Flexible Ureteroscope (U-Scope) is a sterile, single-use, flexible, digital video ureteroscope intended to be used to visualize organs, cavities, and canals in the urinary tract (urethra, bladder, ureter, calyces, and renal papillae) via trans-urethral or per cutaneous access routes. It can also be used in conjunction with endoscopic instruments via its working channel to perform various diagnostic and therapeutic procedures in the urinary tract.
OPCOM medical, a professional endoscope manufacturer from Taiwan that can directly support your success in the market.With our private label service, you can easily launch your own branded medical device without the complexities of in-house development. OPCOM Medical delivers tailor-made solutions in full compliance with FDA 510(k) and ISO 13485.
Discover more on how OPCOM Medical can help you accelerate time-to-market and bring the proven innovation under your own brand.